Surgical Instruments & Implants System
 +
+Spine Instruments System
Surgical System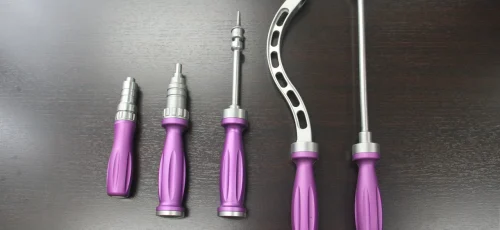 +
+Hip Instruments System 2
Hip Instruments System , Surgical System +
+Hip Prosthesis Implants
Hip Prosthesis Implants , Surgical System +
+Spine Implants System
Spine Implants System , Surgical System +
+Knee Instruments System
Knee Instruments System , Surgical System +
+Hip Instruments System
Hip Instruments System , Surgical System
Surgical Instruments
3D INSTRUMENTATION is specialized in Trauma, Knee, Hip & Spine Instruments. Call us about your manufacturing needs today!

Multi Plate Bender
Surgical Instruments
Mini Plate Plier
Surgical Instruments
Drill Guide
Surgical Instruments
Locking Sleeve
Surgical Instruments
Drill Bits
Surgical Instruments
Big Cutter
Surgical Instruments
L-Wrench
Surgical Instruments
Tibial Nail Instruments
Surgical Instruments
Nail Instruments
Surgical Instruments
Surgical Instruments
Surgical Instruments
Orthopedic Implants
We specialized in manufacturing services to produce Orthopedic Implants. Our areas of expertise include: Nail Implants, Plate Implants & Screw Implants.
PRECISION CNC MACHINING
CNC MILLING
3D Instrumentation utilizes a growing figure of precision multi-axis CNC Milling machines to meet the tolerances required by medical devices and implants. Our expert programmers utilize the Computer Numerical Control (CNC) milling process to maintain tight tolerances and assure manufacturing precision.CNC AUTO LATHE
TORNOS Swiss Type High-Precision Multi-Axis CNC Auto Lathe Turning, up to 9-Axis allows for complex manufacturing.CNC TURNING
Precision multi-axis CNC Turning.GUN DRILL
WIRE CUT & EDM
FINISHING AND ASSEMBLY
- Our specialists will work closely with you to determine the best finishing process for your particular medical device. We offer a wide variety of surface treatments.
ASSEMBLY
EPOXY PAINT
ELECTROPOLISHING
BUFFING & POLISHING
TUMBLING
BEAD BLAST & HYDROHONE
LASER MARKING
LASER ENGRAVING
HOT NITRIC & HOT CITRIC PASSIVATION
TIG WELDING
TITANIUM TYPE III ANODIZING
Titanium Type II Anodizing (Validation in-progress)
QUALITY ASSURANCE
- We understand the importance of manufacturing quality medical devices to your exact specification with:
Validation
Control Plan
Devices Master Record (DMR)
Devices History Record (DHR)
Risk Management (PFMEA)
Gauge Repeatability & Reproducibility (GR&R)
Statistical Process Control & Process Capability Study (SPC & CPK)
ENGINEERING
- Experienced engineering group can examine a part and recommend the most efficient, cost effective solution. Our level of expertise allows us to identify potential failure modes and manage risk. The software application of CAD-CAM for CNC machine programmers, machinists, and R&D engineers. With this, complicated and intricate components will be able to be produced at a cycle time, which is more competitive and simplifying production to retain superior quality.
DESIGN & DEVELOPMENT
Typical system management collaboration
Design verification and validation
Feasibility study and evaluation
Functional prototyping
Design Risk assessment for manufacturing
Design process / fixture for manufacture and assembly
Project management for coordinating
Development of devices master record
Typical system project / product transfer
ISO 13485:2016 Certification
At 3D Instrumentation Sdn Bhd, we understand the importance of quality management systems (QMS) within the medical device industry. That's why we're proud to have earned ISO 13485:2016 certification, an internationally agreed standard that specifies the requirements for a QMS within companies that provide medical devices and related services.
Since 2015, our QMS has been designed to establish and maintain the effectiveness of our processes, ensuring the consistent design, development, production, assembly, and delivery of medical devices that are safe for their intended purpose. We've earned certification from accredited bodies, such as the prestigious Royal Chartership BSI Group, known for its expertise in certification and assessment.
Our ISO 13485:2016 certification demonstrates our long-standing commitment to quality and safety in every aspect of our business. You can trust us to deliver innovative and reliable solutions that meet the highest standards in the medical device industry.
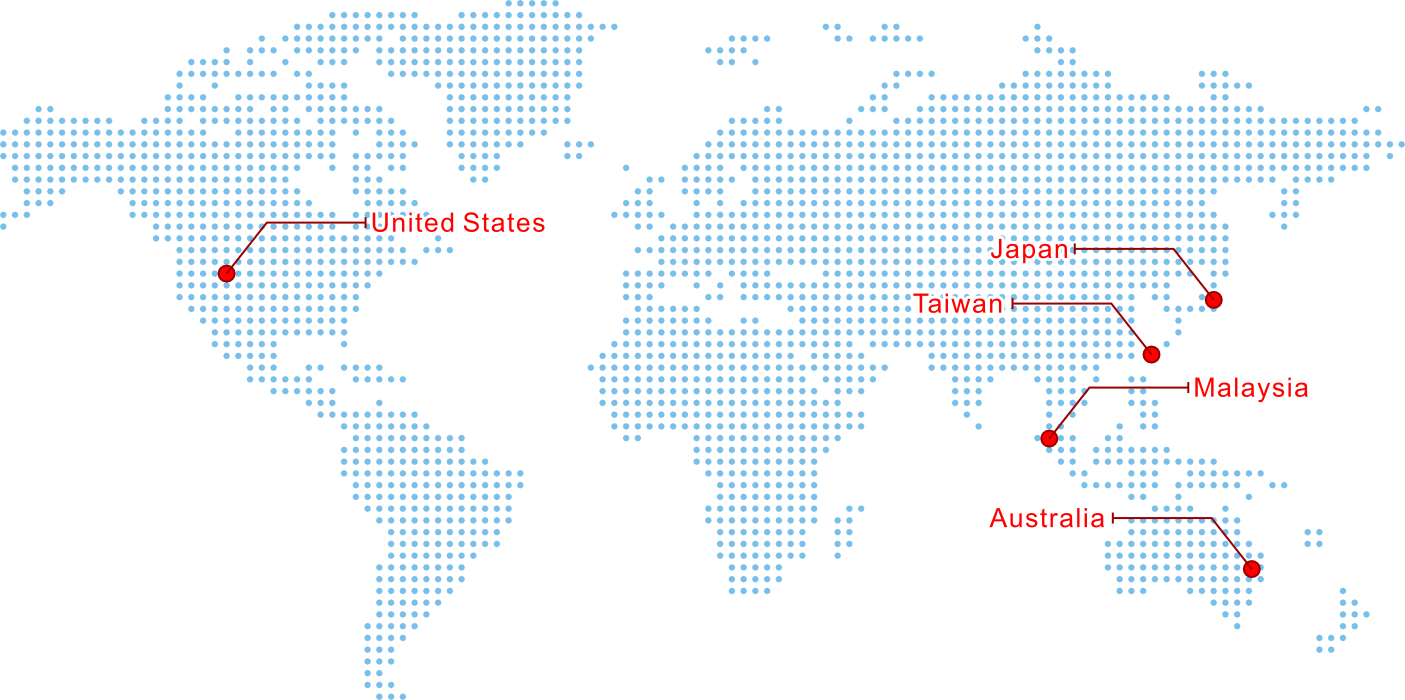
Proudly Serving Customers Worldwide

OUR SERVICES
Specialized Contract Manufacturing

We service the worldwide orthopedic industry with specialized contract manufacturing services to produce surgical instruments, nail implants, plate implants and screw implants of the highest quality from Penang, Malaysia.
does 3D stand for?
- Dynamic - We constantly embrace rapid changes and have the capacity to shift our paradigm through innovation and creativity
- Drive - We have great urge and willingness to pursue our believes and core competency in achieving our target
- Distinction - We will seek our best orthopaedic manufacturing experience to ensure superb efficiency and effectiveness

3D INSTRUMENTATION SDN BHD
3D INSTRUMENTATION is located in Juru Prime industrial Park, Penang, Malaysia.

We feel fortunate to be operating in an industry that holds the power to affect lives in such a profound way and we are proud of the services we offer.

To be Malaysia's market leader in orthopedic medical devices by implementing complete One - Stop Contract Manufacturing Solution.

Maximizing customer Quality and On-time Delivery satisfaction are 3D INSTRUMENTATION’s primary objectives.

- Customer - Exceeding customer’s expectation and confidence.
- Investor / Shareholder - Ensure reasonable profit to stimulate growth.
- Employees - Respect individuals thus providing a great career path.
- Supplier - Being good paymaster & business partners.
- Community - Being good corporate citizen.